Your Ebv quantitative pcr images are ready in this website. Ebv quantitative pcr are a topic that is being searched for and liked by netizens now. You can Find and Download the Ebv quantitative pcr files here. Download all free photos and vectors.
If you’re looking for ebv quantitative pcr pictures information related to the ebv quantitative pcr interest, you have come to the right site. Our site always gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and find more informative video articles and graphics that fit your interests.
Ebv Quantitative Pcr. Epstein-Barr DNA Quant PCR. Positive EBV PCR has been demonstrated in the serum of patients with primary EBV infections and EBV reactivation. It has not been cleared or approved by the. Two hundred fifty nanograms of DNA was used for the real-time PCR assay and the EBV DNA copy numbers per microgram of DNA are shown.
 Realline Ebv Quantitative Bioron Diagnostics From bioron.de
Realline Ebv Quantitative Bioron Diagnostics From bioron.de
Order serological testing Epstein-Barr Viral Ab Panel LAB4584 instead. Clinical Utility EBV is a herpes virus that has been implicated in the development of. The detection and quantitation of Epstein Barr Virus EBV by real-time PCR amplification. Epstein-Barr DNA Quant PCR. DNA was extracted from PBMNC obtained from patients with symptomatic EBV infections or control patients without EBV-related diseases. This test was developed and its performance characteristics.
Epstein-Barr Virus DNA Quantitative Real-Time PCR - Monitoring EBV DNA levels by quantitative PCR in patients at risk of EBV-associated lymphoproliferative disorders may allow timely recognition of virus reactivation and permit installment of antiviral treatment.
The quantitative range of this assay is 26-76 log copiesmL 390-39000000 copiesmL A negative result less than 26 log copiesmL or less than 390 copiesmL does not rule out the presence of PCR inhibitors in the patient specimen or EBV DNA nucleic acid in. If 72 hours centrifuge and freeze the plasma. In the current study we validated five novel quantitative polymerase chain reaction Q-PCR assays targeting disparate but. A patient value of less than 200 EBV DNA copiesmL indicates that the viral load is below the quantitative limit of this assay but does not indicate that the patient is not infected with EBV. The kit contains a standard. Specimens stored at 4C will be accepted up to 72 hours after collection.
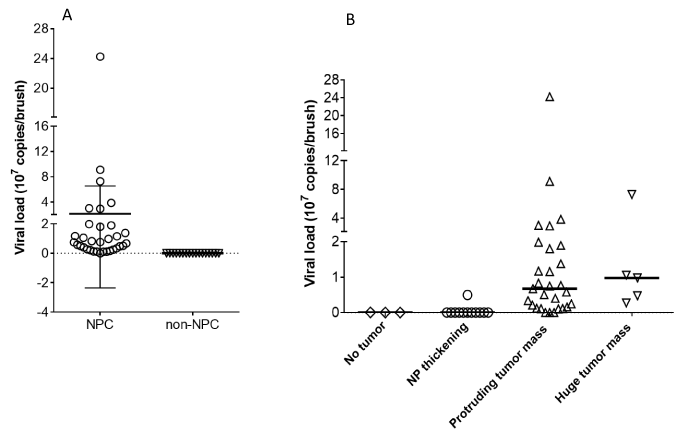 Source: oatext.com
Source: oatext.com
EBV was identified as the cause of CNS infection in 28 patients. Specimens stored at 4C will be accepted up to 72 hours after collection. Log10 EBV DNA Qn PCR. AccuPower EBV Quantitative PCR Kit includes serially diluted Standard Positive Control SPC 15 for the quantification of EBV. This assay does not genotype the virus and consequently does not provide any information with regard to EBV drug resistance.
 Source: researchgate.net
Source: researchgate.net
In the current study we validated five novel quantitative polymerase chain reaction Q-PCR assays targeting disparate but. This assay does not genotype the virus and consequently does not provide any information with regard to EBV drug resistance. Determined by the University of Chicago Medical Center Clinical. If 72 hours centrifuge and freeze the plasma. AccuPower EBV Quantitative PCR Kit test results using clinical samples.
 Source: researchgate.net
Source: researchgate.net
14 with CNSL 10 with encephalitis and 4 with postinfectious neurological complications. H-DiaEBVQ TM is a trusted and easy-to-use molecular diagnostic CE-IVD kit to perform quantitative real-time PCR to detect and measure the viral load of EpsteinBarr virus EBV in total bloodEDTA or plasmacitrateThe kit is used on samples from potentially infected patients by the cytomegalovirus EBV. 1Departments of Otolaryngology-Head and Neck Surgery Centenary Site Rouge Valley Health System Scarborough Ontario Canada. Epstein-Barr Virus EBV infects nearly all humans and then persists for the life of the host. Epstein-Barr Virus EBV DNA quantification is based upon the real-time PCR amplification and detection of EBV genomic DNA.
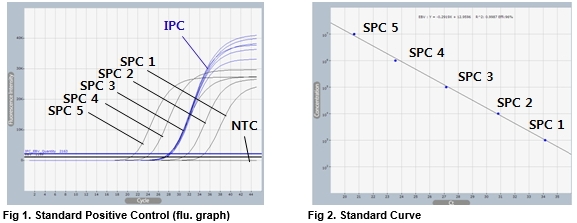 Source: eng.bioneer.com
Source: eng.bioneer.com
The presence of variations in EBV DNA sequences are monitored in the real-time PCR assay and if detected the sample is retested using reagents to alternate amplification targets to verify EBV detection and quantification. EBV was identified as the cause of CNS infection in 28 patients. This 5 nuclease-based real-time PCR assay amplifies a specific region of the EBV genome for detection and quantification of EBV. Four Quantification Standards ensure accurate EBV viral load measurement. This test is only used as an aid in monitoring EBV-related disease.
 Source: researchgate.net
Source: researchgate.net
The presence of variations in EBV DNA sequences are monitored in the real-time PCR assay and if detected the sample is retested using reagents to alternate amplification targets to verify EBV detection and quantification. Does not require this test to. We measured EBV DNA by quantitative PCR and EBV mRNA by RT-PCR in the CSF in patients with EBV-associated neurological disorders. Clinical Utility EBV is a herpes virus that has been implicated in the development of. In some people who later develop cancer EBV DNA is present within malignant cells and circulates at el-evated levels in the plasma.
 Source: semanticscholar.org
Source: semanticscholar.org
More recently quantitative real-time PCR has been developed for assessing copy levels of DNA of CMV EBV and BKV in blood specimens obtained from patients receiving solid-organ transplants. Order serological testing Epstein-Barr Viral Ab Panel LAB4584 instead. AccuPower EBV Quantitative PCR Kit test results using clinical samples. Food and Drug Administration. Epstein-Barr Virus EBV infects nearly all humans and then persists for the life of the host.
 Source: researchgate.net
Source: researchgate.net
This is a quantitative molecular test with a linear range of 200-2000000. Food and Drug Administration. H-DiaEBVQ TM is a trusted and easy-to-use molecular diagnostic CE-IVD kit to perform quantitative real-time PCR to detect and measure the viral load of EpsteinBarr virus EBV in total bloodEDTA or plasmacitrateThe kit is used on samples from potentially infected patients by the cytomegalovirus EBV. A patient value of less than 200 EBV DNA copiesmL indicates that the viral load is below the quantitative limit of this assay but does not indicate that the patient is not infected with EBV. The quantitative range of this assay is 26-76 log copiesmL 390-39000000 copiesmL A negative result less than 26 log copiesmL or less than 390 copiesmL does not rule out the presence of PCR inhibitors in the patient specimen or EBV DNA nucleic acid in.
 Source: eng.bioneer.com
Source: eng.bioneer.com
This 5 nuclease-based real-time PCR assay amplifies a specific region of the EBV genome for detection and quantification of EBV. Positive EBV PCR has been demonstrated in the serum of patients with primary EBV infections and EBV reactivation. Epstein-Barr DNA Quant PCR. AccuPower EBV Quantitative PCR Kit includes serially diluted Standard Positive Control SPC 15 for the quantification of EBV. The ready-to-use EBV R-GENE molecular detection kit measures EBV viral load in DNA extracts from different clinical samples.
 Source: biomerieux-diagnostics.com
Source: biomerieux-diagnostics.com
The kit contains a standard. Log10 EBV DNA Qn PCR. Ng RH1 Ngan R Wei WI Gullane PJ Phillips J. Determined by the University of Chicago Medical Center Clinical. Clinical Utility EBV is a herpes virus that has been implicated in the development of.
 Source: researchgate.net
Source: researchgate.net
A patient value of less than 200 EBV DNA copiesmL indicates that the viral load is below the quantitative limit of this assay but does not indicate that the patient is not infected with EBV. It has not been cleared or approved by the. Quantitation of EBV DNA IUmL is achieved by amplifying a standard curve. EBV qualitative and quantitative PCR. 1Departments of Otolaryngology-Head and Neck Surgery Centenary Site Rouge Valley Health System Scarborough Ontario Canada.
 Source: researchgate.net
Source: researchgate.net
Epstein-Barr DNA Quant PCR. Determined by the University of Chicago Medical Center Clinical. The kit contains a standard. This test is only used as an aid in monitoring EBV-related disease. The EBER1 probes are labeled with different reporter dyes.
 Source: researchgate.net
Source: researchgate.net
Four Quantification Standards ensure accurate EBV viral load measurement. Epstein-Barr DNA Quant PCR. The detection and quantitation of Epstein Barr Virus EBV by real-time PCR amplification. Log10 EBV DNA Qn PCR. Epstein-Barr Virus DNA Quantitative Real-Time PCR - Monitoring EBV DNA levels by quantitative PCR in patients at risk of EBV-associated lymphoproliferative disorders may allow timely recognition of virus reactivation and permit installment of antiviral treatment.
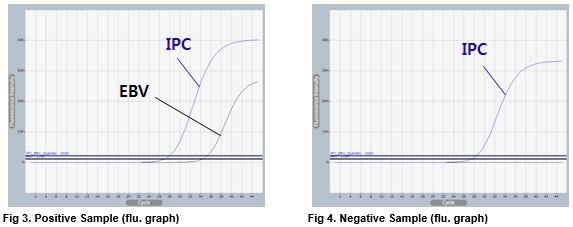 Source: eng.bioneer.com
Source: eng.bioneer.com
AccuPower EBV Quantitative PCR Kit includes serially diluted Standard Positive Control SPC 15 for the quantification of EBV. If 72 hours centrifuge and freeze the plasma. The presence of variations in EBV DNA sequences are monitored in the real-time PCR assay and if detected the sample is retested using reagents to alternate amplification targets to verify EBV detection and quantification. The quantitative range of this assay is 26-76 log copiesmL 390-39000000 copiesmL A negative result less than 26 log copiesmL or less than 390 copiesmL does not rule out the presence of PCR inhibitors in the patient specimen or EBV DNA nucleic acid in. It has not been cleared or approved by the.
 Source: researchgate.net
Source: researchgate.net
Recently EBV PCR has been added as a diagnostic tool. Commercial qualitative and quantitative PCRbased assays Qualitative EBV Viral Detect and Quantitative EBV Viral Quant BioSource International Inc Camarillo CA were performed according to the manufacturers instructions for the detection and quantification of EBVspecific amplicons from. A patient value of less than 200 EBV DNA copiesmL indicates that the viral load is below the quantitative limit of this assay but does not indicate that the patient is not infected with EBV. Order serological testing Epstein-Barr Viral Ab Panel LAB4584 instead. The kit contains a standard.
 Source: bioron.de
Source: bioron.de
Epstein-Barr DNA Quant PCR. Commercial qualitative and quantitative PCRbased assays Qualitative EBV Viral Detect and Quantitative EBV Viral Quant BioSource International Inc Camarillo CA were performed according to the manufacturers instructions for the detection and quantification of EBVspecific amplicons from. It is not appropriate for the diagnosis of mononucleosis. The presence of variations in EBV DNA sequences are monitored in the real-time PCR assay and if detected the sample is retested using reagents to alternate amplification targets to verify EBV detection and quantification. If 72 hours centrifuge and freeze the plasma.
 Source: eng.sansure.com.cn
Source: eng.sansure.com.cn
14 with CNSL 10 with encephalitis and 4 with postinfectious neurological complications. Recently EBV PCR has been added as a diagnostic tool. Positive EBV PCR has been demonstrated in the serum of patients with primary EBV infections and EBV reactivation. Quantitation of EBV DNA IUmL is achieved by amplifying a standard curve. Epstein-Barr Virus EBV infects nearly all humans and then persists for the life of the host.
 Source: ejcancer.com
Source: ejcancer.com
Log10 EBV DNA Qn PCR. Quantitation of EBV DNA IUmL is achieved by amplifying a standard curve. This test was developed and its performance characteristics. The detection and quantitation of Epstein Barr Virus EBV by real-time PCR amplification. Specimens stored at 4C will be accepted up to 72 hours after collection.
 Source: benthamopen.com
Source: benthamopen.com
The kit employs IPC in all wells to confirm correct PCR amplification. Order serological testing Epstein-Barr Viral Ab Panel LAB4584 instead. Quantitation of EBV DNA IUmL is achieved by amplifying a standard curve. 14 with CNSL 10 with encephalitis and 4 with postinfectious neurological complications. We measured EBV DNA by quantitative PCR and EBV mRNA by RT-PCR in the CSF in patients with EBV-associated neurological disorders.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ebv quantitative pcr by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






