Your Metal halogen exchange images are available in this site. Metal halogen exchange are a topic that is being searched for and liked by netizens today. You can Get the Metal halogen exchange files here. Download all royalty-free vectors.
If you’re looking for metal halogen exchange pictures information linked to the metal halogen exchange interest, you have visit the right site. Our site always provides you with suggestions for seeking the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
Metal Halogen Exchange. Metal-halogen exchange In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. Metal-catalysed halogen exchange reactions of aryl halides Tom D. 101039b818155a Aryl halides are common synthetic targets themselves and also highly versatile synthetic intermediates. The kinetics of reaction of n-butyllithium with substituted bromobenzenes in hexane solution.
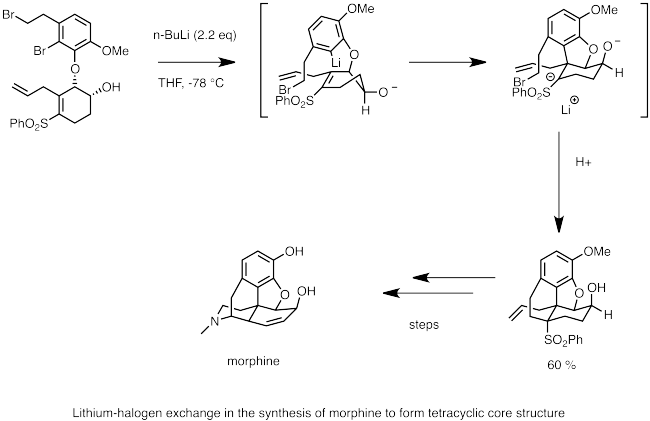 Metal Halogen Exchange Wikipedia From en.wikipedia.org
Metal Halogen Exchange Wikipedia From en.wikipedia.org
Metal-halogen exchange reactions of polybromoimidazoles B. Show transcribed image text. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. Subsequent studies of the reactions of PhLi with fluorobenzene led to the first example of a reaction proceeding via a benzyne intermediate Wittig G. A reaction or reaction mechanism step in which a bond between a halogen atom and another atom often a carbon is changed into a bond between a metal atom and another atom. The first equivalent is used for the exchange and the second equivalent reacts with the t-BuI produced to form isobutene isobutane and lithium iodide.
101039b818155a Aryl halides are common synthetic targets themselves and also highly versatile synthetic intermediates.
The first equivalent is used for the exchange and the second equivalent reacts with the t-BuI produced to form isobutene isobutane and lithium iodide. H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K. Expert Answer 100 1 rating Previous question Next question Transcribed Image Text from this Question. Halogen-Metal Exchange ceRBr ceRLi rightleftharpoons ceRLi ceRBr The equilibrium in these reactions favors formation of the organometallic compound with the metal attached to the more electronegative ceR group. The reaction commonly involves the use of electropositive metals Li Na Mg and organochlorides bromides and iodides. Metalhalogen exchange reactions of mono- and poly-halogenoimidazoles B.
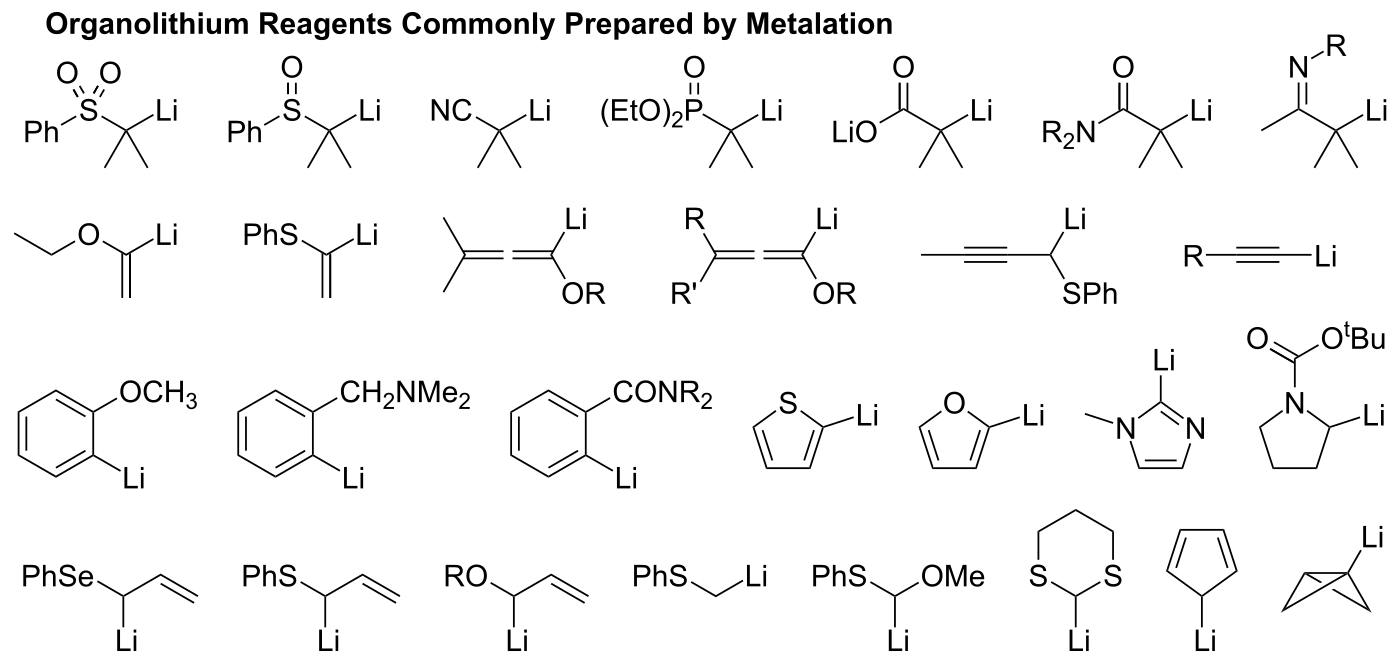 Source: wikiwand.com
Source: wikiwand.com
Metal-halogen exchange In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. Metalhalogen exchange reactions of mono- and poly-halogenoimidazoles B. The yields of the newly formed Grignard and the final product are substantially improved in comparison to the identical reaction without a. The method is mainly used in the preparation of organolithium compounds derived from unreactive halides. In order to determine whether the exchange reaction did occur the reaction mixture was quenched with various electrophiles at low.
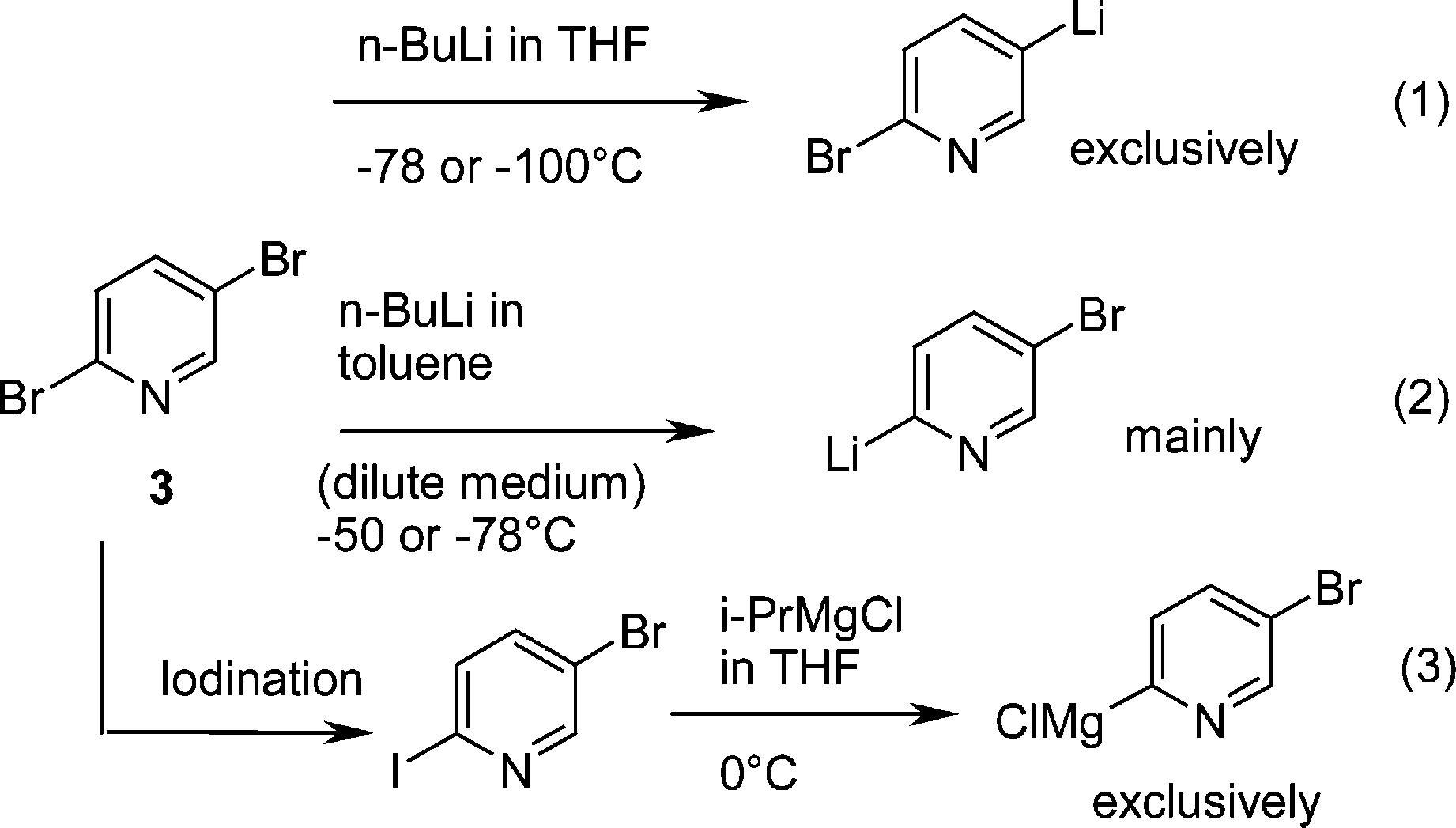 Source: finder-articles.com
Source: finder-articles.com
A reaction or reaction mechanism step in which a bond between a halogen atom and another atom often a carbon is changed into a bond between a metal atom and another atom. In the presence of the tridentate ligand both the metalhalogen exchange and the reaction with the electrophile can be performed at 10 to 25 C. Metal-halogen exchange In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. Expert Answer 100 1 rating Previous question Next question Transcribed Image Text from this Question. H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
Sheppard Received 15th October 2008 Accepted 11th December 2008 First published as an Advance Article on the web 30th January 2009 DOI. Metal-halogen exchange reactions of polybromoimidazoles B. Generalized halogen -metal exchange. The yields of the newly formed Grignard and the final product are substantially improved in comparison to the identical reaction without a. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X.
 Source: organic-chemistry.org
Source: organic-chemistry.org
Metal-halogen exchange In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. In order to determine whether the exchange reaction did occur the reaction mixture was quenched with various electrophiles at low. Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 both PDF and HTML across all institutions and individuals. Reagents via metal-halogen exchange has not been widely used until recently. Halogen Magnesium exchange allows the Synthesis of organomagnesium reagents with many more functional groups than previously thought.
 Source: www2.chem.wisc.edu
Source: www2.chem.wisc.edu
The kinetics of reaction of n-butyllithium with substituted bromobenzenes in hexane solution. Halogen Magnesium exchange allows the Synthesis of organomagnesium reagents with many more functional groups than previously thought. 1 1983 735 DOI. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. Metal-catalysed halogen exchange reactions of aryl halides Tom D.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. A reaction or reaction mechanism step in which a bond between a halogen atom and another atom often a carbon is changed into a bond between a metal atom and another atom. The yields of the newly formed Grignard and the final product are substantially improved in comparison to the identical reaction without a. In the above reaction the Grignard reagent cannot simply replace phenyllithiun some Mg cases have been reported for other halides but Li is the stalwart metal. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Utilising the racemic phenoxide ligand 5566-tetramethyl-33-di-tert-butyl-11-biphenyl-22-diol rac-BIPHEN-H2 the dialkyl sodium magnesiates rac-BIPHENNa2MgBu2TMEDA2 3 and rac-BIPHENNa2MgBu2PMDETA2 4 have been synthesised. Preliminary studies of the mechanism of metal-halogen exchange. The mild reaction conditions are the key for assuring high functional group tolerance. Sheppard Received 15th October 2008 Accepted 11th December 2008 First published as an Advance Article on the web 30th January 2009 DOI. 101039b818155a Aryl halides are common synthetic targets themselves and also highly versatile synthetic intermediates.
Source: chemicalforums.com
The kinetics of reaction of n-butyllithium with substituted bromobenzenes in hexane solution. Metal-halogen exchange In organometallic chemistry metalhalogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. Preliminary studies of the mechanism of metal-halogen exchange. Subsequent studies of the reactions of PhLi with fluorobenzene led to the first example of a reaction proceeding via a benzyne intermediate Wittig G. Magnesium-Halogen Exchange Chem 115 Jason Brubaker Review.
 Source: chem.ucla.edu
Source: chem.ucla.edu
Sheppard Received 15th October 2008 Accepted 11th December 2008 First published as an Advance Article on the web 30th January 2009 DOI. Knochel and coworkers have demonstrated the functional-group tolerance of magnesium-halogen exchange which is now the method of choice for the preparation of highly functionalized organomagnesium reagents. Preliminary studies of the mechanism of metal-halogen exchange. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. Metal-halogen exchange reactions of polybromoimidazoles B.
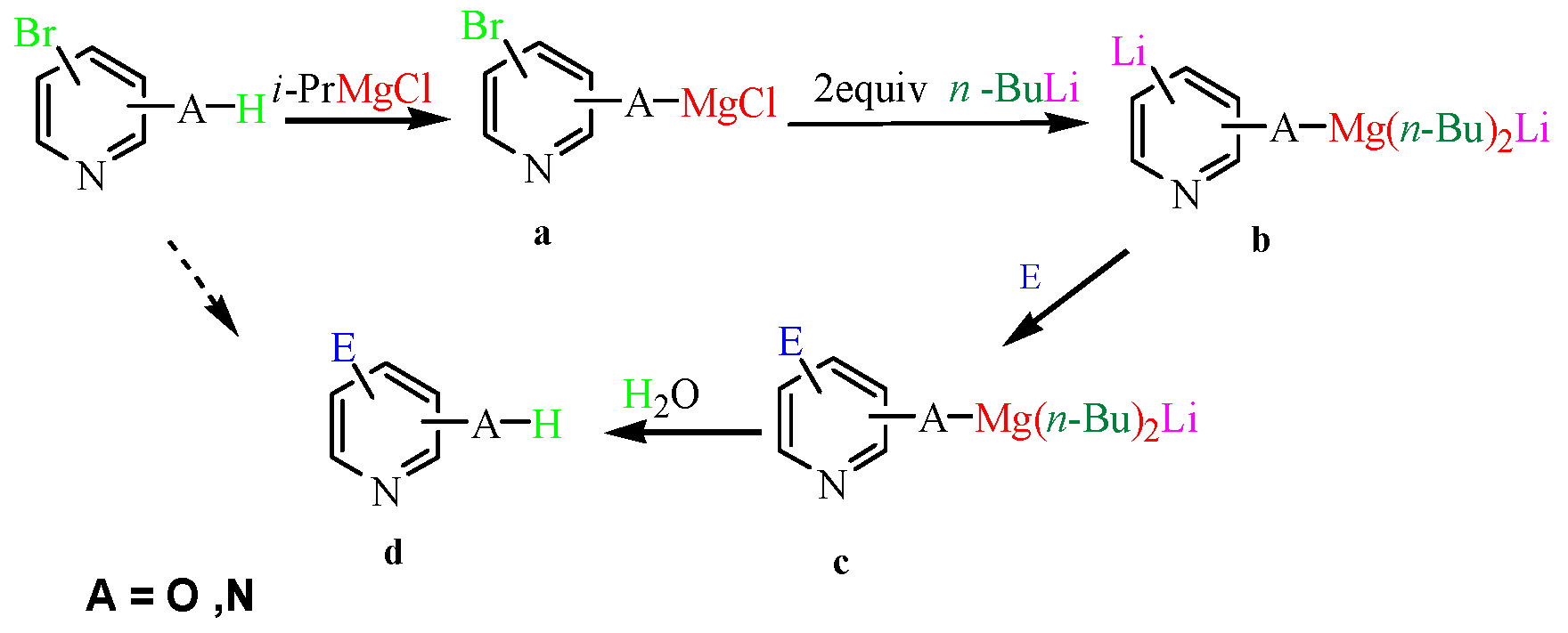 Source: mdpi.com
Source: mdpi.com
Knochel and coworkers have demonstrated the functional-group tolerance of magnesium-halogen exchange which is now the method of choice for the preparation of highly functionalized organomagnesium reagents. Reagents via metal-halogen exchange has not been widely used until recently. H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K. Expert Answer 100 1 rating Previous question Next question Transcribed Image Text from this Question. Halogenmetal exchange was attempted at -100 o C in tetrahydrofuran using n-butyllithium as the organometallic exchange reagent.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Halogen-Metal Exchange ceRBr ceRLi rightleftharpoons ceRLi ceRBr The equilibrium in these reactions favors formation of the organometallic compound with the metal attached to the more electronegative ceR group. Metal-halogen exchange reactions of polybromoimidazoles B. 1 1987 1445 DOI. Reagents via metal-halogen exchange has not been widely used until recently. 101039b818155a Aryl halides are common synthetic targets themselves and also highly versatile synthetic intermediates.
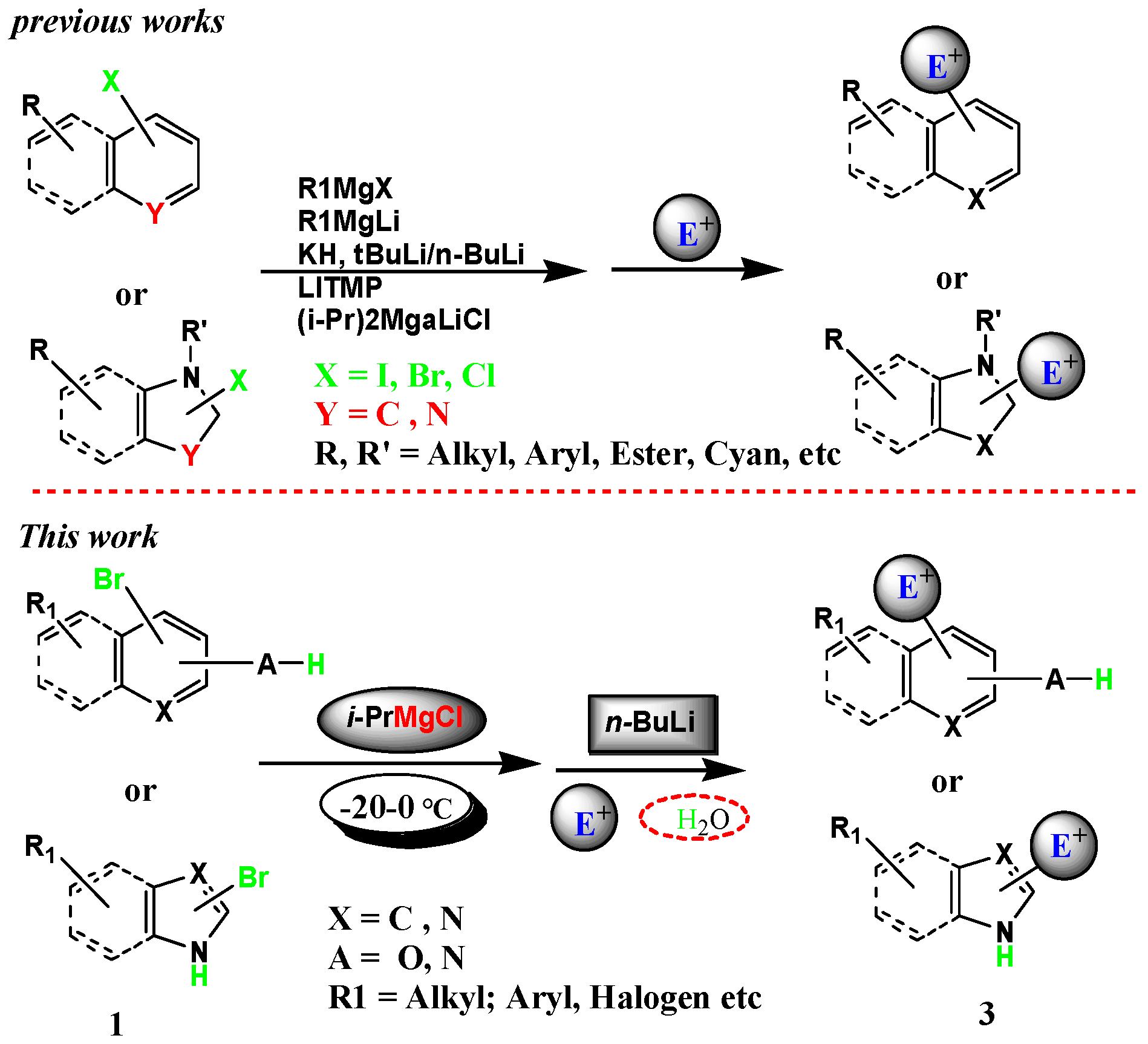 Source: mdpi.com
Source: mdpi.com
1 1987 1445 DOI. The reaction commonly involves the use of electropositive metals Li Na Mg and organochlorides bromides and iodides. Show transcribed image text. Metalhalogen exchange reactions of mono- and poly-halogenoimidazoles B. Magnesium-Halogen Exchange Chem 115 Jason Brubaker Review.
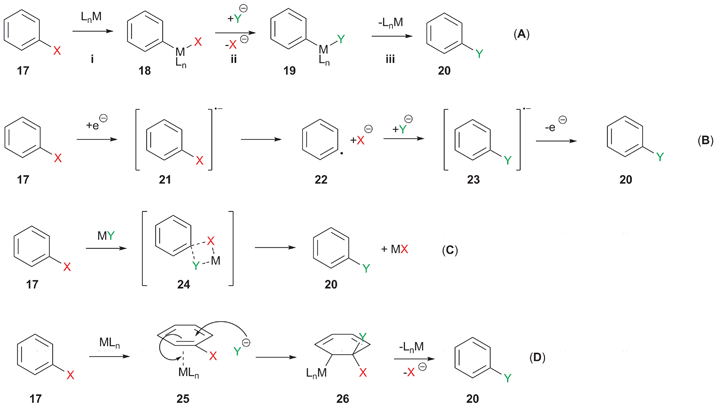 Source: pubs.rsc.org
Source: pubs.rsc.org
Halogenmetal exchange was attempted at -100 o C in tetrahydrofuran using n-butyllithium as the organometallic exchange reagent. Sheppard Received 15th October 2008 Accepted 11th December 2008 First published as an Advance Article on the web 30th January 2009 DOI. Metal-halogen exchange reactions of polybromoimidazoles B. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X. Utilising the racemic phenoxide ligand 5566-tetramethyl-33-di-tert-butyl-11-biphenyl-22-diol rac-BIPHEN-H2 the dialkyl sodium magnesiates rac-BIPHENNa2MgBu2TMEDA2 3 and rac-BIPHENNa2MgBu2PMDETA2 4 have been synthesised.
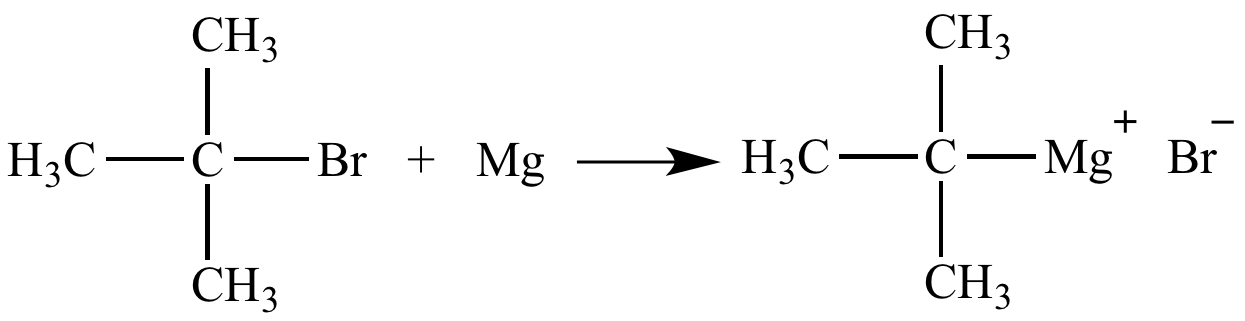 Source: chem.ucla.edu
Source: chem.ucla.edu
Halogen-Metal Exchange ceRBr ceRLi rightleftharpoons ceRLi ceRBr The equilibrium in these reactions favors formation of the organometallic compound with the metal attached to the more electronegative ceR group. Generalized halogen -metal exchange. Subsequent studies of the reactions of PhLi with fluorobenzene led to the first example of a reaction proceeding via a benzyne intermediate Wittig G. Metal-catalysed halogen exchange reactions of aryl halides Tom D. What is the side product for this reaction.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The method is mainly used in the preparation of organolithium compounds derived from unreactive halides. Magnesium-Halogen Exchange Chem 115 Jason Brubaker Review. The mild reaction conditions are the key for assuring high functional group tolerance. The method is mainly used in the preparation of organolithium compounds derived from unreactive halides. 1 1983 735 DOI.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The reaction commonly involves the use of electropositive metals Li Na Mg and organochlorides bromides and iodides. Today this reversible lithium-halogen exchange reaction can be formulated as R L i R X K R L i R X. Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. Utilising the racemic phenoxide ligand 5566-tetramethyl-33-di-tert-butyl-11-biphenyl-22-diol rac-BIPHEN-H2 the dialkyl sodium magnesiates rac-BIPHENNa2MgBu2TMEDA2 3 and rac-BIPHENNa2MgBu2PMDETA2 4 have been synthesised. H Br OEt H H Li OEt 11 eq n-BuLi H Et2O 80 C Lau K.
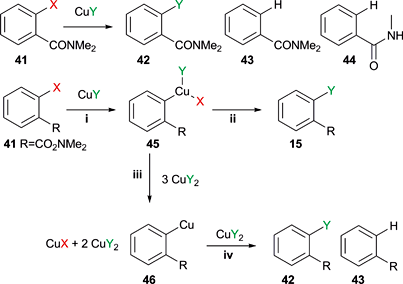 Source: pubs.rsc.org
Source: pubs.rsc.org
Lithium-halogen exchange reactions using t-BuLi typically employ two or more equivalents of t-BuLi. Reagents via metal-halogen exchange has not been widely used until recently. Show transcribed image text. Metal-catalysed halogen exchange reactions of aryl halides Tom D. Knochel and coworkers have demonstrated the functional-group tolerance of magnesium-halogen exchange which is now the method of choice for the preparation of highly functionalized organomagnesium reagents.
 Source: researchgate.net
Source: researchgate.net
The kinetics of reaction of n-butyllithium with substituted bromobenzenes in hexane solution. Bimetallic sodium magnesiates have been employed in metal-halogen exchange for the first time. 101039b818155a Aryl halides are common synthetic targets themselves and also highly versatile synthetic intermediates. The first equivalent is used for the exchange and the second equivalent reacts with the t-BuI produced to form isobutene isobutane and lithium iodide. A general example for such a reaction is given below BuLi n-butyl lithium.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title metal halogen exchange by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






