Your Unfolded protein response review images are ready in this website. Unfolded protein response review are a topic that is being searched for and liked by netizens today. You can Get the Unfolded protein response review files here. Download all free photos.
If you’re looking for unfolded protein response review pictures information related to the unfolded protein response review keyword, you have come to the ideal blog. Our site frequently gives you hints for downloading the highest quality video and picture content, please kindly hunt and locate more enlightening video content and images that fit your interests.
Unfolded Protein Response Review. In response to stress eg accumulation of misfolded proteins the ER triggers the unfolded protein response UPR a complex and conserved signalling pathway that is mediated by three ER transmembrane sensor proteins. Mediated by the unfolded protein response UPR a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load. Recent evidence implicates the participation of adaptive responses to stress within the endoplasmic reticulum ER in the disease process via a pathway known as the unfolded protein response UPR.
 Unfolded Protein Response In Leukemia From Basic Understanding To Therapeutic Opportunities Trends In Cancer From cell.com
Unfolded Protein Response In Leukemia From Basic Understanding To Therapeutic Opportunities Trends In Cancer From cell.com
This is the biochemical basis for many ER storage diseases in which folding-incompetent proteins accumulate in the ER 1415. The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or in the event of chronic damage induce apoptotic cell death. The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or in the event of chronic damage induce apoptotic cell death. Here we review the findings suggesting. To cope with the stress cells activate an intracellular signaling pathway the unfolded protein response UPR. In mammalian cells UPR is a complex signaling program mediated by three ER transmembrane receptors.
Activated in response to stress in the endoplasmic reticulum ER.
Accumulation of unfolded proteins in the endoplasmic reticulum ER due to suboptimal environmental conditions triggers a response called the unfolded protein response UPR which induces a set of genes that elevate protein folding capacity in the ER. Accumulation of unfolded proteins in the endoplasmic reticulum ER due to suboptimal environmental conditions triggers a response called the unfolded protein response UPR which induces a set of genes that elevate protein folding capacity in the ER. A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load. In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. The unfolded protein response UPR is a highly conserved pathway that allows the cell to manage endoplasmic reticulum ER stress that is imposed by the secretory demands associated with. In this review we briefly summarize principles of protein folding and molecular chaperone function important for a mechanistic understanding of UPR-signaling events.
 Source: researchgate.net
Source: researchgate.net
Here we review the findings suggesting. In this scenario the UPR has three aims. Initially to restore normal function of the cell by halting protein translation degrading misfolded proteins. In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. Exhaustion of the capacity of this protein folding machinery by.
 Source: tocris.com
Source: tocris.com
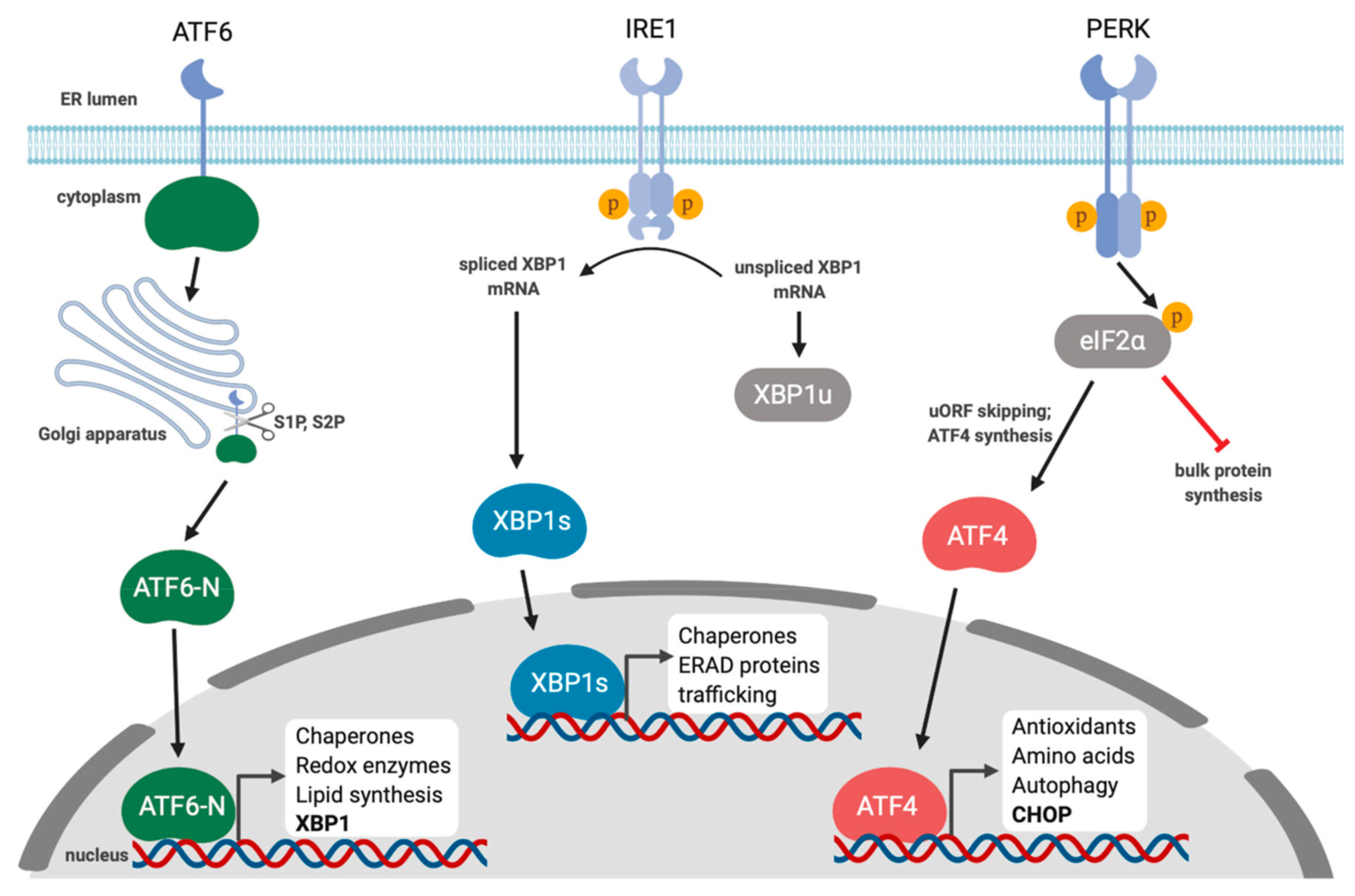
The unfolded protein response is a cellular stress response related to the endoplasmic reticulum stress. In response to stress eg accumulation of misfolded proteins the ER triggers the unfolded protein response UPR a complex and conserved signalling pathway that is mediated by three ER transmembrane sensor proteins. Inositolrequiring enzyme 1 alpha IRE1α protein kinase RNAlike ER kinase PERK and activating transcription factor 6 ATF6. In vivo protein folding requires a complex ER-resident protein folding machinery. The unfolded protein response is a cellular stress response related to the endoplasmic reticulum stress.
 Source: researchgate.net
Source: researchgate.net
The UPR is activated in response to an accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum. Here we review the findings suggesting. In this review we briefly summarize principles of protein folding and molecular chaperone function important for a mechanistic understanding of UPR-signaling events. These pathways are called the unfolded protein response UPR. The Unfolded Protein Response.
 Source: researchgate.net
Source: researchgate.net
In vivo protein folding requires a complex ER-resident protein folding machinery. These pathways are called the unfolded protein response UPR. Activated in response to stress in the endoplasmic reticulum ER. Initially to restore normal function of the cell by halting protein translation degrading misfolded proteins. In response to stress eg accumulation of misfolded proteins the ER triggers the unfolded protein response UPR a complex and conserved signalling pathway that is mediated by three ER transmembrane sensor proteins.
 Source: researchgate.net
Source: researchgate.net
In this scenario the UPR has three aims. The Unfolded Protein Response. A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load. In vivo protein folding requires a complex ER-resident protein folding machinery. In this review we briefly summarize principles of protein folding and molecular chaperone function important for a mechanistic understanding of UPR-signaling events.
 Source: researchgate.net
Source: researchgate.net
Recent evidence implicates the participation of adaptive responses to stress within the endoplasmic reticulum ER in the disease process via a pathway known as the unfolded protein response UPR. In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. Activated in response to stress in the endoplasmic reticulum ER. The unfolded protein response UPR is a highly conserved pathway that allows the cell to manage endoplasmic reticulum ER stress that is imposed by the secretory demands associated with. It has been found to be conserved between all mammalian species as well as yeast and worm organisms.
 Source: science.sciencemag.org
Source: science.sciencemag.org
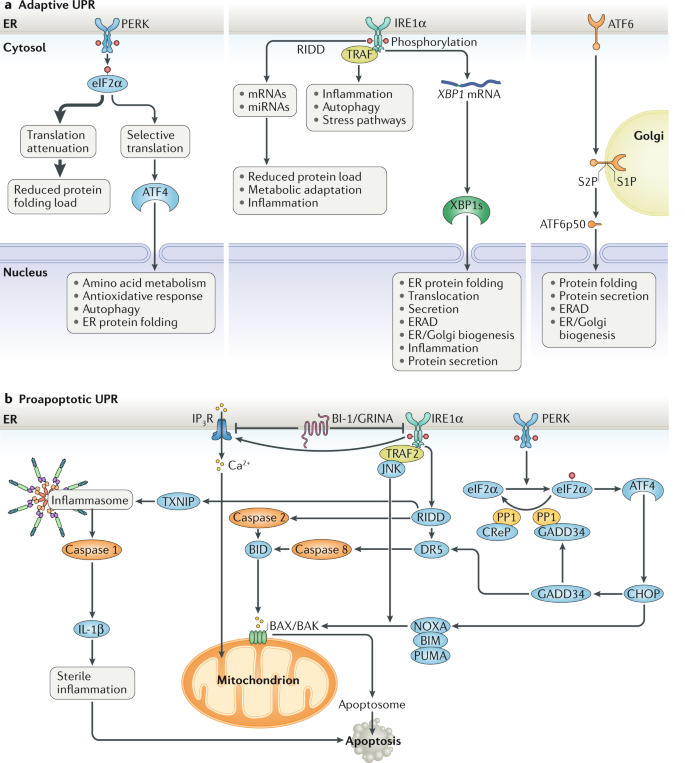
The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or in the event of chronic damage induce apoptotic cell death. Cells respond to ER stress by activating the unfolded protein response UPR. Inositolrequiring enzyme 1 alpha IRE1α protein kinase RNAlike ER kinase PERK and activating transcription factor 6 ATF6. The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or in the event of chronic damage induce apoptotic cell death. These pathways are called the unfolded protein response UPR.
 Source: cell.com
Source: cell.com
The unfolded protein response UPR comprises a network of signalling pathways that reprogramme transcription translation and protein modifications to relieve the load of unfolded or misfolded. In mammalian cells UPR is a complex signaling program mediated by three ER transmembrane receptors. Accumulation of unfolded proteins in the endoplasmic reticulum ER due to suboptimal environmental conditions triggers a response called the unfolded protein response UPR which induces a set of genes that elevate protein folding capacity in the ER. A collection of phylogenetically conserved signaling pathways collectively termed the unfolded protein response UPR monitors conditions in the ER sensing an insufficiency in the ERs. The unfolded protein response UPR is an intracellular signaling pathway that is.
 Source: sciencedirect.com
Source: sciencedirect.com
In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. UPR can effectively cope with stress by reducing the amount of misfolded protein overload in this subcellular organelle. In this scenario the UPR has three aims. The UPR is initially protective but in situations of prolonged unresolved stress the UPR can lead to the apoptotic death of the stressed cell. Here we review the findings suggesting.
 Source: researchgate.net
Source: researchgate.net
In this scenario the UPR has three aims. In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. To cope with the stress cells activate an intracellular signaling pathway the unfolded protein response UPR. The unfolded protein response UPR is a highly conserved pathway that allows the cell to manage endoplasmic reticulum ER stress that is imposed by the secretory demands associated with. A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load.
 Source: researchgate.net
Source: researchgate.net
The Unfolded Protein Response. A review of the mammalian unfolded protein response. The unfolded protein response is a cellular stress response related to the endoplasmic reticulum stress. Activating transcription factor 6 ATF6 inositol requiring kinase 1 IRE1 and doublestranded RNAactivated protein kinase PKR. In this review we briefly summarize principles of protein folding and molecular chaperone function important for a mechanistic understanding of UPR-signaling events.
 Source: mdpi.com
Source: mdpi.com
To cope with the stress cells activate an intracellular signaling pathway the unfolded protein response UPR. These pathways are called the unfolded protein response UPR. The unfolded protein response UPR is a highly conserved pathway that allows the cell to manage endoplasmic reticulum ER stress that is imposed by the secretory demands associated with. Proteins requiring post-translational modifications such as N-linked glycosylation are processed in the endoplasmic reticulum ER. To cope with the stress cells activate an intracellular signaling pathway the unfolded protein response UPR.
 Source: researchgate.net
Source: researchgate.net
Inositolrequiring enzyme 1 alpha IRE1α protein kinase RNAlike ER kinase PERK and activating transcription factor 6 ATF6. Mediated by the unfolded protein response UPR a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. Activated in response to stress in the endoplasmic reticulum ER. Proteins requiring post-translational modifications such as N-linked glycosylation are processed in the endoplasmic reticulum ER. It has been found to be conserved between all mammalian species as well as yeast and worm organisms.
 Source: sciencedirect.com
Source: sciencedirect.com
The Unfolded Protein Response. The UPR transmits information about protein folding status to the nucleus and cytosol to adjust the protein folding capacity of the cell or in the event of chronic damage induce apoptotic cell death. ER proteostasis surveillance is mediated by the unfolded protein response UPR a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. A collection of phylogenetically conserved signaling pathways collectively termed the unfolded protein response UPR monitors conditions in the ER sensing an insufficiency in the ERs. The unfolded protein response UPR is a highly conserved pathway that allows the cell to manage endoplasmic reticulum ER stress that is imposed by the secretory demands associated with.
 Source: fertstert.org
Source: fertstert.org
The unfolded protein response UPR comprises a network of signalling pathways that reprogramme transcription translation and protein modifications to relieve the load of unfolded or misfolded. When misfolded proteins accumulate above a critical threshold this sets in motion a signal transduction pathway called the unfolded protein response UPR within the cell to restore homeostasis. Activating transcription factor 6 ATF6 inositol requiring kinase 1 IRE1 and doublestranded RNAactivated protein kinase PKR. Mediated by the unfolded protein response UPR a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. Exhaustion of the capacity of this protein folding machinery by.
 Source: cell.com
Source: cell.com
These pathways are called the unfolded protein response UPR. When misfolded proteins accumulate above a critical threshold this sets in motion a signal transduction pathway called the unfolded protein response UPR within the cell to restore homeostasis. In vivo protein folding requires a complex ER-resident protein folding machinery. The UPR is initially protective but in situations of prolonged unresolved stress the UPR can lead to the apoptotic death of the stressed cell. The unfolded protein response UPR is an intracellular signaling pathway that is.
 Source: cell.com
Source: cell.com
The unfolded protein response UPR is an intracellular signaling pathway that is. A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load. In this review after introducing the Unfolded Protein Response we will summarize current findings on the involvement of ER stress in the progression of leukemia and discuss the potential therapeutic effects of UPR activation or repression in these pathologies. The unfolded protein response is a regulatory mechanism that enhances the expression of proteins involved in the function of the endoplasmic reticulum ER including ER chaperones as well as components of ER-associated degradation when eukaryotic cells increase the production of secretory proteins and the capacity of the ER function is overwhelmed. Recent evidence implicates the participation of adaptive responses to stress within the endoplasmic reticulum ER in the disease process via a pathway known as the unfolded protein response UPR.
 Source: nature.com
Source: nature.com
Inositolrequiring enzyme 1 alpha IRE1α protein kinase RNAlike ER kinase PERK and activating transcription factor 6 ATF6. In this Review we discuss recent advances in the mechanisms of UPR signaling its regulation and its relevance to pathological conditions. The UPR is initially protective but in situations of prolonged unresolved stress the UPR can lead to the apoptotic death of the stressed cell. Cells respond to ER stress by activating the unfolded protein response UPR. Initially to restore normal function of the cell by halting protein translation degrading misfolded proteins.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title unfolded protein response review by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






